Services

Project Management
Bodiewox Clinical Research, our project managers endeavor that our team maintain both study timeline and budget limits throughout the lifecycle of the trial, while ensuring effective communication with key stakeholders throughout the trial.
Clinical Trial Monitoring
Bodiewox Clinical Research, oversees clinical trial processes, ensuring that it is conducted, recorded, and reported in accordance with the protocol, SOPs, The Principles of GCP, and the Medicines for Human Use Regulations
Data Management
Bodiewox Clinical Research, experts can assist in implementing patient-centric, alternative study models – integrating digital health technologies while maintaining data integrity.

GCP Compliance Auditing
Bodiewox Clinical Research, provides GCP auditing expertise which can help you to meet international ethical and scientific standards of Good Clinical Practice.

Investigator Payments
Bodiewox Clinical Research, provides timely and accurate Investigator payments which are critical for keeping sites motivated and driving site performance.

Regulatory Submissions
Bodiewox Clinical Research, complies with the laws and regulations of every aspects of the drug development process while ensuring that all partner sites are always compliant

Hybrid Clinical Solutions
Bodiewox Clinical Research, provides hybrid clinical solutions that are evidence-based specializing in wellness treatments and home visits all through agile monitoring solutions.

Clinical Supplies Management
Bodiewox Clinical Research, utilizes leading edge technologies to ensure that the safest clinical supplies are used while integrating programs that ensure competitive sourcing, packaging and labeling of all materials including biological samples and effective logistic management to provide optimal value for all trials
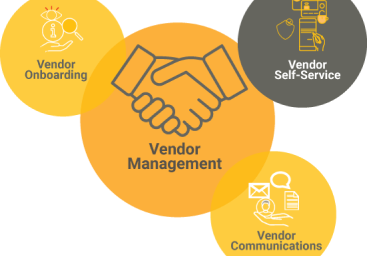
Vendor Management
Bodiewox Clinical Research, enables organizations to control costs, drive service excellence and mitigate risks to gain increased value from our vendors throughout the trial life cycle.

Risk Management
Bodiewox Clinical Research, engages in continuous oversight and monitoring of sites to minimize potential risks to patients and ensure data integrity.

Quality Management
Bodiewox Clinical Research, has a team of experts that oversee all activities and tasks needed to maintain a desired level of excellence.

Mobile Nursing Services
Bodiewox Clinical Research, supports the medical care of people in the comfort, familiarity, and security of their homes.
Site & Patient Solutions:
Bodiewox Clinical Research, also provides the following:
- Site Management Services;
- Site Identification Services;
- Patient Recruitment Services;
- PI and Staff Recruitment Services.
